Building a Total Supply Chain Service for Regenerative Medicine
The Alfresa Group's Take on Regenerative Medicine
We aim to develop storage and transportation facilities for regenerative medicine products nationwide and build a regenerative medicine supply chain service across the entire Alfresa Group.

Nationwide Expansion of Storage and Transportation Facilities for Regenerative Medicine Products
Regenerative medicine product repository
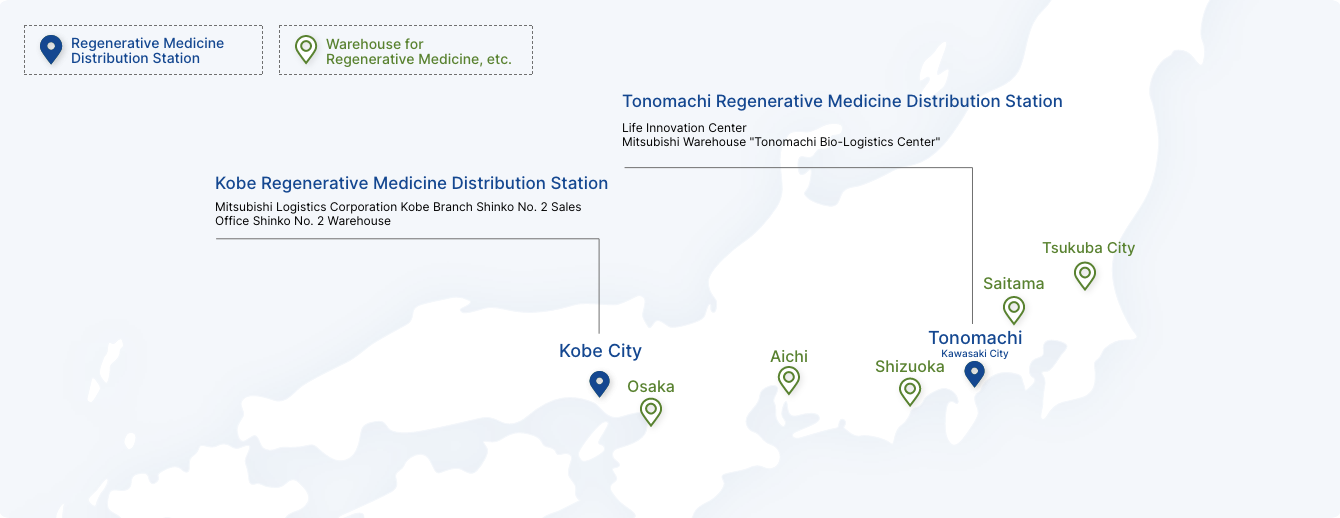
Regenerative medicine products require a more stringent management system than regular pharmaceuticals, including ultra-low temperature storage and detailed product traceability. Alfresa has established "regenerative medicine product warehouses" equipped with storage tanks using liquid nitrogen capable of supporting temperatures of -150°C, ultra-low temperature freezers capable of supporting temperatures of -80°C, and systems for loading/unloading and inventory management at its distribution centers in Saitama, Aichi, Osaka, Shizuoka, and Tsukuba, strengthening its system for safely and securely storing and transporting regenerative medicine products.



Regenerative Medicine Distribution Station
We have signed a service agreement with Mitsubishi distribution Corporation and established Regenerative Medicine Distribution Stations in Kawasaki City, Kanagawa Prefecture and Kobe City, Hyogo Prefecture.
The Regenerative Medicine Distribution Station will have a storage and transportation environment similar to that of a regenerative medicine product warehouse, and will be able to handle storage and transportation operations according to customer requests.
It can be particularly useful for the distribution of products in the clinical trial stage, autologous cell-based regenerative medicine products, and other challenging products. They also serve as manufacturing bases (packaging, labeling, storage) or sales bases for pharmaceutical companies' regenerative medicine products.
From cell extraction and processing to the manufacture of regenerative medicine products
Cell Resources is established
Alfresa established Cell Resources in April 2022 to contribute to the stable supply of regenerative medicine products.
Cell Resources CorporationWith the philosophy of "bringing the hope of regenerative medicine to everyone," the company operates two manufacturing bases: Koriyama Cell Processing Center and Tonomachi Cell Processing Center. We aim to promote the business of providing domestic raw materials (master cells) and the manufacturing of autologous and allogeneic cell processed products and regenerative medicine products.
-
1. Provision of domestic raw materials (master cells)
We manufacture cell raw materials (master cells) using tissues and cells provided with the support of affiliated medical care institutions in Japan, and use them to develop regenerative medicine products. In addition to providing the service to bio-venture companies, research institutes, university hospitals, and medical care institutions, we are also considering offering this service to CMO companies.
-
2. Manufacturing of autologous allogeneic cell-processed products and regenerative medicine products
Manufacturing business of autologous and allogeneic cell processed products and regenerative medicine products that we manufacture cells derived from the clients themselves or from all other people according to the needs of the clients who wish to manufacture the products.